Nanjing Leads Biolabs Co., Ltd (“Leads Biolabs” or “company”), announced that its proprietary fully human anti-LAG-3 monoclonal antibody, LBL-007, has been recently granted permission to clinical trial by NMPA following submitting the clinical trial application in May, 2019.
Due to the low response rate of existing tumor immunotherapy, combined therapies based on multiple biomarkers and targets have been extensively explored. Leads Biolabs adopted this approach in developing LBL-007, an anti-LAG-3 antibody with potential to be combined with anti-PD-1 therapy to treat patients resistant or not responding to anti-PD-1 therapy.
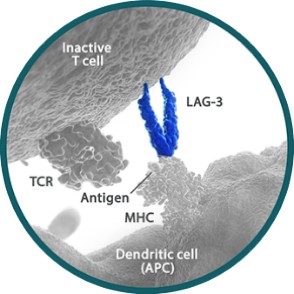
About LAG-3 protein
Lymphocyte activation gene 3 (LAG-3) is a kind of type I transmembrane protein expressed on activated T cells and natural killer cells, negatively regulating the immunologic functions of these cells by binding to MHC-II. LAG-3 is expressed in antigen-activated T cells or depleted T cells (such as tumor-infiltrating T cells), and is significantly highly expressed in tumor-infiltrating T cells resistant to PD-1 therapy than those not resistant to PD-1 therapy.
In September 2017, BMS announced data of the Phase 1/2a clinical trial of combination therapy of LAG-3 / PD-1 among melanoma patients not responding to immunotherapy or developed resistance to immunotherapy, at the annual meeting of the European Society for Medical Oncology (ESMO). The results showed that among patients with LAG-3 expression level greater than 1%, 18% responded to the combination therapy, while among those with LAG-3 expression level less than 1%, only 5% responded to the combination therapy.
About LBL-007
LBL-007 is a novel fully human IgG4 anti-LAG-3 monoclonal antibody isolated from a human phage library. By binding with human LAG-3 protein, LBL-007 could block the binding of LAG-3 protein and its ligands which cast inhibitory effect on T cells. Thus, the exhausted T cells could restore its immunologic function to inhibit tumor growth.
Following the great success of PD-1, LAG-3 is widely expected to be another important target for tumor immunotherapy. Currently, a number anti-LAG-3 agents are being evaluated against solid tumors in clinical trials.
Dr. Kang Xiaoqiang, founder, CEO and chair of the board of Leads Biolabs, remarked on the clinical trial approval,
“LBL-007 is the first proprietary antibody developed by Leads Biolabs. The acquisition of NMPA clinical trial approval is an important milestone in the development of our company. It’s the result of concerted efforts of our team, and also acknowledgement of our innovation ability. The approval allows us to move LBL-007 quickly to clinical stage.”
Another co-founder of Leads Biolabs, Dr. Lai Shoupeng, is an expert in commercialization of biologic drugs. He has long engaged in process development, management of GMP facilities as well as project management.
Introduced by Dr Kang Xiaoqiang,
“Leads Biolabs is an innovation-driven biopharmaceutical company with a proprietary pipeline consisting novel mono-targeted antibodies, bispecific antibodies and fusion protein drug projects, focusing on cancer immunotherapy and other fields with great unmet needs. In the next few years, we expect to receive one or two clinical trial approvals every year.”
In this May, Leads Biolabs announced cooperation with Pneuma Respiratory, Inc., a developer of proprietary breath-activated digital inhaler (BDI), granting Pneuma an exclusive license to develop Leads Biolabs' panel of immuno-oncology monoclonal antibodies and fusion protein molecules for the pulmonary delivery. As Chinese pharmaceutical companies usually acted as patent licensees in the past, this transaction where Leads Biolabs acted as a patent licensor attracted considerable attention within the industry.
By far, Leads Biolabs has completed three rounds of financing and has recently launched a new round of financing with target of 200 million RMB, the proceeds of which would be primarily used for the phase II trial of LBL-007, two phase I trials as well as other early stage projects.